By Greenyn Biotechnology Co., Ltd.
TAIPEI and TAICHUNG, Aug. 27, 2024 /PRNewswire/ — Modern lifestyle is often associated with irregular routines, high-fat/high-salt diets and refined foods as well as less physical activities, which increase the incidence and prevalence of the diseases of civilization.
Metabolic syndrome encompasses issues like abnormal blood lipids, blood sugar, and abdominal obesity (central obesity). Insulin resistance is the primary cause of metabolic syndrome. Prolonged poor blood sugar control can easily lead to diabetes and even other chronic diseases. According to statistics from Ministry of Health, Labour and Welfare, the number of diabetes patients in Japan has exceeded 10 million, with an increasing trend as the population ages. Driven by this substantial demand, bitter melon extract emerged, leading to their popularity in the market.
Potential Plant Insulin
Bitter melon (Momordica charantia Linn.), a member of the Cucurbitaceae family, is a traditional Chinese herbal medicine. Many studies have confirmed that bitter melon contains various blood sugar-lowering components, primarily including terpenes, phytosterols, and peptides. There are many related products on the market extracted from bitter melon (see Table 1). The mechanisms of action range from improving insulin sensitivity, inhibiting glucose-degrading enzymes, to directly binding with insulin receptors(3). With various blood sugar regulation mechanisms, bitter melon has gained significant attention in the field of blood sugar control. However, research shows that if specific component structures are not extracted or if the product is only following market trends and not adequately utilized, over time, ineffective feedback from consumers may lead to market saturation, causing high-quality products to be overlooked.
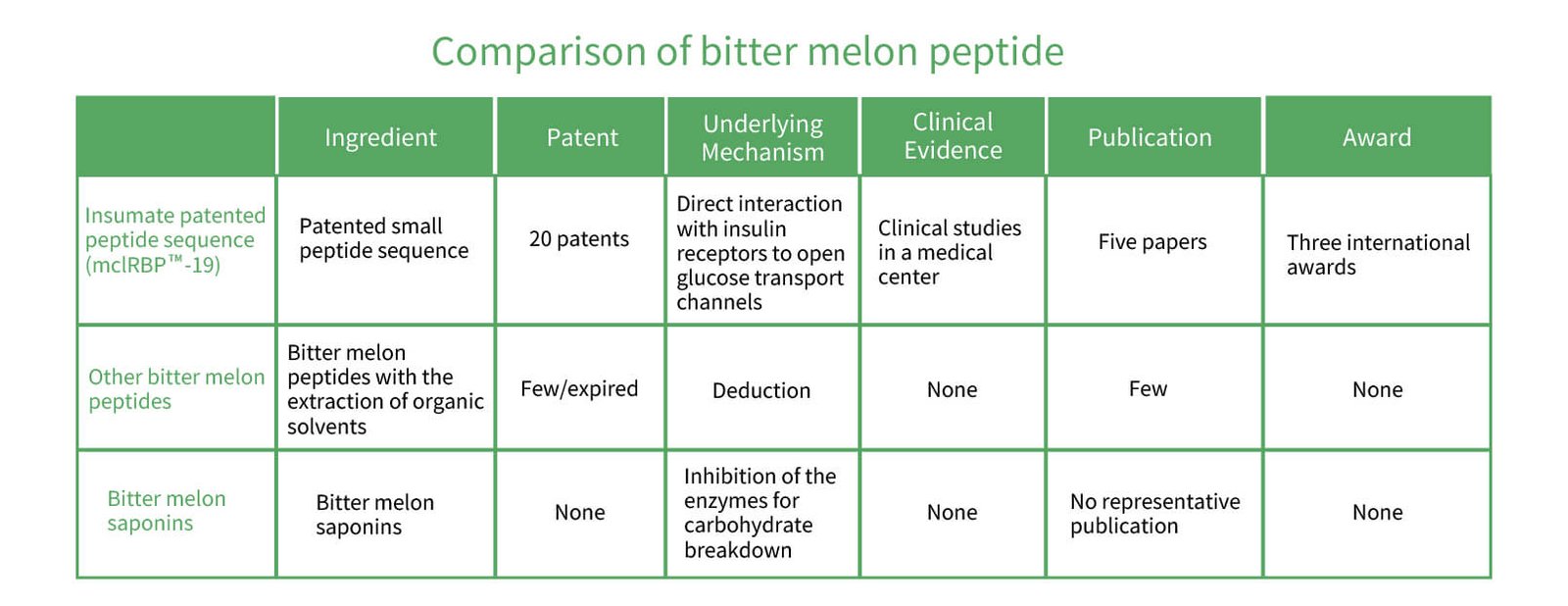
Research on Commercial Bitter Melon Health Ingredients
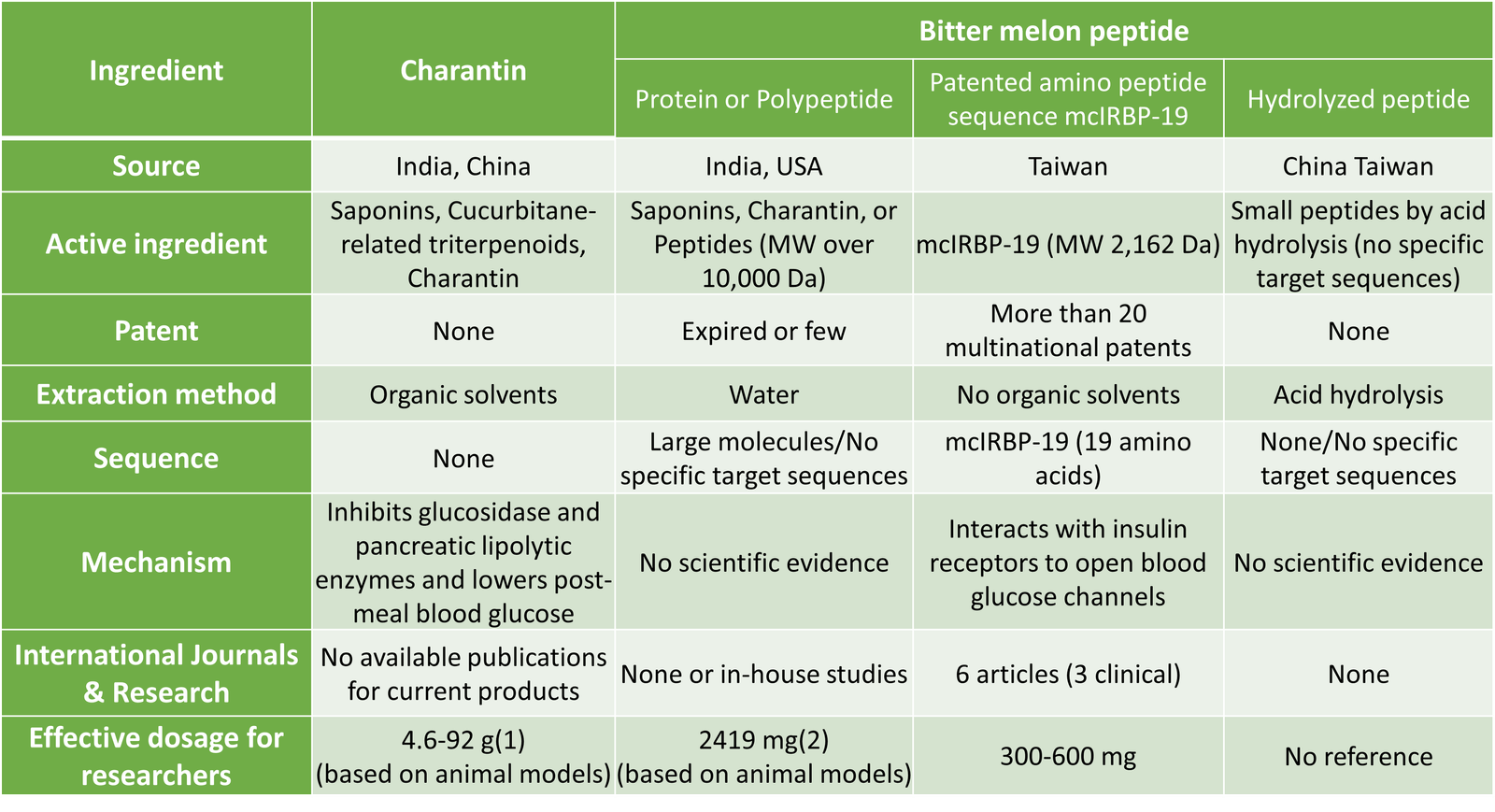
Research on Patented Amino Peptide Sequence mcIRBP-19
In an environment saturated with products claiming to contain bitter melon peptides, many of these lack scientific validation of their functionality or physiological mechanisms. The market is chaotic, with products of varying quality. Due to the complex mechanism of blood sugar regulation, there is rarely a substance that can substitute for insulin in regulating blood sugar. Greenyn Biotechnology has conducted in-depth research on bitter melon, focusing on the essential nature of the plant. They have verified that the specific amino peptide sequence mcIRBP-19, consisting of 19 amino acids, is a rare natural substance that can directly bind to the diabetes-causing target – the insulin receptor. They completed structural sequencing and quantitative analysis, providing scientific evidence that repeatedly confirms its unique physiological function. Additionally, they have conducted several in vivo studies, which are detailed as follows:
Regulating Blood Sugar and Assisting in the Treatment of Diabetes
Diabetes is a hard to cure, and its alarming annual increase rate of 25,000 cases is not the only cause for concern. The complications it leads to, such as retinopathy, neuropathy, and cardiovascular diseases, are even more significant.
Through animal experiments analyzing fasting blood sugar and post-meal blood sugar regulation capabilities, it was found that the patented amino peptide sequence 19 (mcIRBP-19) can reduce fasting blood sugar by 50% and glycosylated hemoglobin by 38%. Additionally, it maintains a balanced insulin level in the body. Furthermore, it prevents a significant increase in post-meal blood sugar, rapidly regulating blood sugar within 2 hours. Its exceptional ability to stabilize blood sugar can significantly reduce the occurrence of complications caused by diabetes, such as retinopathy.
Human clinical studies have shown (4) that the patented amino peptide sequence 19 (mcIRBP-19) can assist in significantly reducing fasting blood sugar and glycosylated hemoglobin within 12 weeks for individuals who have shown insufficient improvement with conventional diabetes medication. It can also effectively improve the quality of life for patients (5). For sub-healthy individuals with diabetes, mcIRBP-19 can help regulate fasting blood sugar, stabilize glycated hemoglobin, and prevent the onset of diabetes. These clinical trials have verified the potential efficacy of this ingredient
Reducing Body Fat, Blood Lipids, and Anti-Inflammation
In addition to unstable blood sugar, metabolic syndrome also involves issues with abnormal blood lipids, central obesity, and chronic inflammation. Animal experiments (6) have shown that the patented amino peptide sequence 19 (mcIRBP-19) can regulate fatty acid metabolism and genes related to fatty liver. It effectively reduces body fat percentage by 25.5%, significantly decreasing white fat in the liver. This helps prevent the occurrence of fatty liver and avoids liver damage. Furthermore, chronic inflammation, much like an internal fire, can lead to various diseases, including cancer. The patented sequence 19 peptide can effectively reduce the expression of inflammatory factors in various organs, thus preventing the onset of chronic diseases.
Conclusion
The human body is like a machine. When metabolic syndrome occurs, it’s like screws gradually loosening. Continued operation can easily lead to machine failure, resulting in conditions such as chronic diseases and cancer. At such times, we urgently need a helper who can tighten the screws.
The structure of mcIRBP-19, with the potential of a plant-derived insulin, is extracted using patented technology. The functional 19 amino acids are fully sequenced. Through a series of animal and clinical experiments, it has been confirmed to stabilize both pre-meal and post-meal blood sugar levels, reduce body fat and blood lipids, and achieve anti-inflammatory effects while providing adjunctive treatment for diabetes. This not only brings a ray of hope to diabetes patients, preventing the deterioration of their condition, but also takes into account their quality of life. It offers everyone a safe and effective natural preventive health option for daily life.
Reference
1. Wang HY, et al. Food Chem Toxicol. (2014) 69:347-356.
2. Dallas LC, et al. J Med Food. (2011) 14:1496-1504.
3. Lo HY, et al. J Agric Food Chem. (2013) 61:2461-2468.
4. Hsu PK, et al. Nutrients (2020) 12:1252.
5. Pan F, et al. Risk Manag Healthc Policy. (2020) 13:2219-2226.
6. Patent Invention No. I580690, Republic of China.
LINE TODAY:https://today.line.me/hk/v2/article/vX6yBX8