Among chronic diseases, the health problems caused by diabetes are particularly pervasive, and growing at an alarming rate. According to data released by the International Diabetes Federation, 463 million people were living with diabetes at the end of 2019, with related medical expenses of US$760 billion. Each year, 4.2 million people die from diabetes and its complications. With insufficient action, these numbers will continue to spiral. China, Southeast Asia, and the Western Pacific Region account for 35% of all diabetics, one third of all confirmed cases.
Dr. Yang Yi-Tien of Chung Shan Medical University Hospital conducted the randomized double-blind clinical trial, which confirmed that Patented Peptide Sequence: mcIRBPTM-19 had outstanding results on diabetic patients who undertook adjuvant therapy. The trial was conducted on 29 diabetics who were long-term patients of hypoglycemic drugs of less effectiveness. Without breaking routine drug therapy, the diabetic trials were conducted for 3 months, concluding that fasting blood sugar and glycosylated hemoglobin in the therapy group were significantly reduced. Their liver function, kidney function, and hemoglobin remained unchanged. There were no side effects of hypoglycemia and allergic gastrointestinal discomfort, which usually occur to users of oral hypoglycemic drugs. As for the control group, there was no noticeable difference. The clinical results are being submitted to an International Journal.
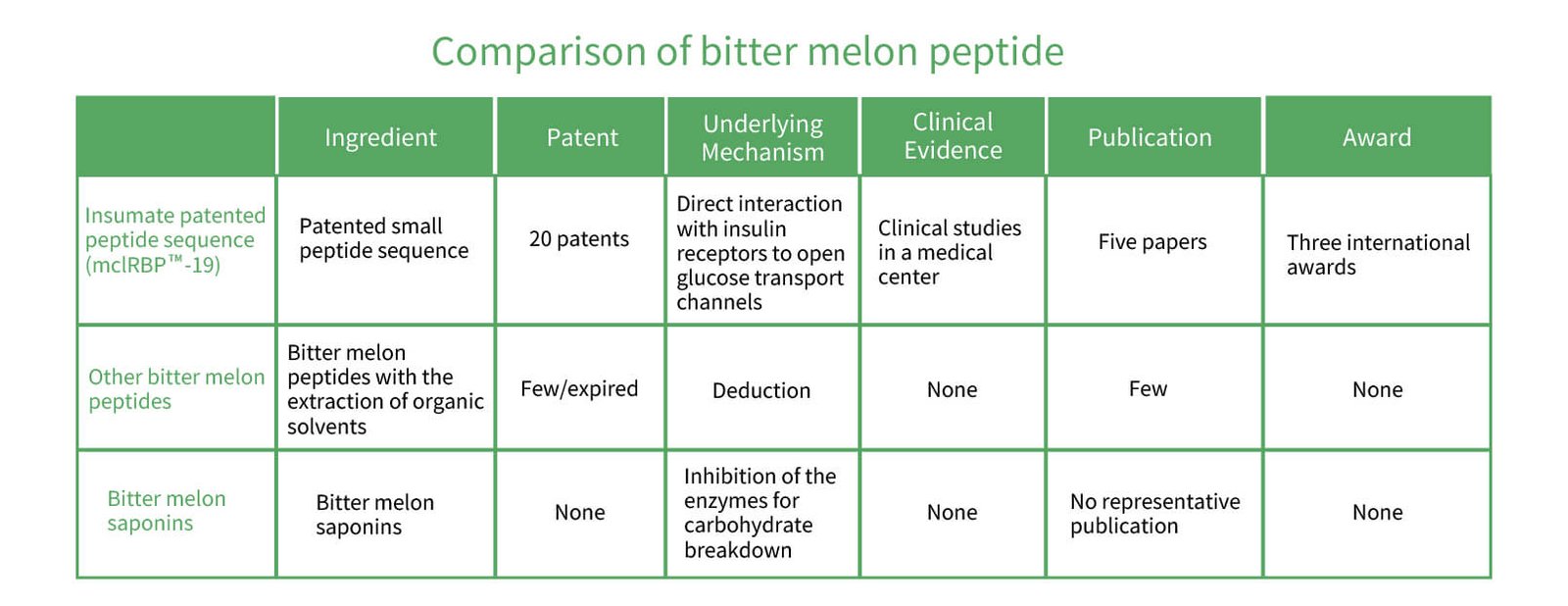
The unique structure of Patented Peptide Sequence: mcIRBPTM-19 allows for use as a pathogenic target. These results have been published in the Science Citation Index (SCI) journals. Dr. Hsu Pang-kuei, chief of research and development at Greenyn Biotechnology, has been conducting research for more than a decade. “After many years, we discovered that mcIRBPTM -19 has a hypoglycemic effect on insulin-like biological activities and can bind insulin receptors. Without stimulating insulin secretion, mcIRBPTM-19 can participate in the body’s carbohydrate metabolism and regulate blood sugar. Besides insulin, mcIRBPTM-19 has been scientifically confirmed as a natural product able to bind insulin receptors to regulate blood sugar,” said Dr. Hsu. Yet another clinical trial reported that 142 diabetics who were long-term users for hypoglycemic drugs took mcIRBPTM -19 for 3 months, and their blood sugar indexes and glycosylated hemoglobin were well regulated. These results were published in the world-famous Journal of Nutrition.
Insumate®: mcIRBPTM-19 functions to regulate blood sugar, processing at low temperatures with high activity, and passing toxicity tests. This biotechnology innovation has helped Greenyn Biotechnology in obtaining 19 patent certificates and recognition from the world’s largest discovery exhibitions, the Discovery and New Products Expo (INPEX) in Pittsburgh, Pa, USA; International Discovery Exhibition of Geneva, Switzerland; and the Nuremberg International Expo (IENA) in Germany. An extensive summary of mcIRBPTM-19 has also been published in the Scientific Citation Index (SCI) journal. Presently, many well-known international process manufacturers use ingredients provided by Greenyn Biotechnology to produce health foods. Greenyn Biotechnology attributes these achievements to its utmost efforts in recent years.